Radioactivity in consumer products
The use of radioactive substances in consumer products is restricted by radiation legislation. However, some products may contain small quantities of radioactive substances that may have been added for properties such as heat resistance, refractive index or colour. For example, radioactivity may be present in some watches, compasses, lamps and jewellery.
Properties can be achieved by the addition of artificial radioactive substances such as promethium-147, krypton-85 or americium-241, or natural radioactive substances such as uranium-238, uranium-235, thorium-232 and their decay products and potassium-40.
The deliberate use of radioactive substances in foodstuffs, cosmetic products, jewelry, and other similar consumer goods and toys is prohibited. The transport, import and export of consumer goods containing radioactive substances is also prohibited (Radiation Act, Section 68).
Regulation of radiating products
Depending on the radioactivity of the product, it will determine how they are regulated and whether there are requirements or restrictions on their use, import or disposal as waste. This page contains information on radioactive consumer products and some other radioactive substances that consumers may encounter
Uranium and thorium are nuclear materials subject to international safeguards and holders are generally subject to extensive accounting and reporting obligations. However, small quantities of uranium and thorium in utility items and equipment are, in principle, excluded from international safeguards. However, the import and possession of semi-finished products and raw materials containing uranium and thorium require notification to the Radiation and Nuclear Safety Authority (STUK). STUK will provide the notifier with a copy of the declaration for the customs authorities, if necessary. The copy is marked to indicate that the import does not require a license under the Nuclear Energy Act
Customs notifies STUK if radiating products or materials are detected at the Finnish borders.
Some foods can be irradiated to improve their shelf life, such as spices. Irradiated foods are safe.
More information on safe handling of food can be found on the Finnish Food Authority's website.
Radioactive substances in consumer products
Radioactive substances such as radium, tritium and sometimes promethium-147 may have been used in the luminous colours of clocks, compasses, sights and instruments. Old clocks may still use radium as a luminous colour, but in the 1970s the gamma-emitting radium was replaced by beta emitters, tritium (H-3) and promethium-147. Today, compasses and clocks generally use glowing dyes that are not radioactive, but tritium and promethium-147 are still used in some clocks.
The most common radioactive substance used in light colours is tritium, which is used as an encapsulated gas in so-called tritium lamps, for example in self-illuminating navigation devices and exit signs for aircraft, ships and public spaces. Tritium lamps may also be used as a light source for liquid crystal display clocks, dials or key rings. Light sources containing tritium and promethium do not expose the user to radiation when the protective glass of the watch, compass or meter is intact.





The table clock hands, and clock face components contain radium, with a surface dose rate of 1.2 microsievert per hour. A wristwatch containing radium dates from the turn of the 1950s and 1960s, with a surface dose rate of about 8 micorsievert per hour.
For more information [email protected]
Some lamps use small amounts of radioactive material to improve the lamp's ignition and other properties. These gas discharge lamps are mainly used in professional and special lighting. Applications include large public spaces such as stadiums, railway stations or shopping centres. One accepted application is also the use of xenon lamps in cars.
The radioactive substances used in these lamps are thorium (Th-232), krypton (Kr-85) or tritium (H-3).
The amount of radioactive material in a lamp typically ranges from a few becquerels to a few hundred becquerels. The radioactivity in a lamp cannot be detected by a standard radiation detector and it does not expose users to radiation.
Some gas lanterns or oil lamps in the past have used thorium oxide in the mantle because of the pleasant colour of the light. During combustion and especially during the ignition process, thorium and its decomposition products are released into the air in small quantities. In this case, a small internal radiation exposure through inhalation is possible. Radiation exposure may also occur, for example, from careless handling of the gas lantern mantle when replacing a fragile, burnt-out mantle with a new one. In this case, thorium and its decomposition products may be inhaled with the dust. There are also gas mantles on the market today that do not contain thorium nitrate. Even then, care must be taken when handling used gas mantles to avoid inhalation of toxic substances contained in the mantle.


Gas discharge lamps containing radioactive krypton (Kr-85, half-life about 10 years) with an activity of about 130 and 250 becquerels in 2012.
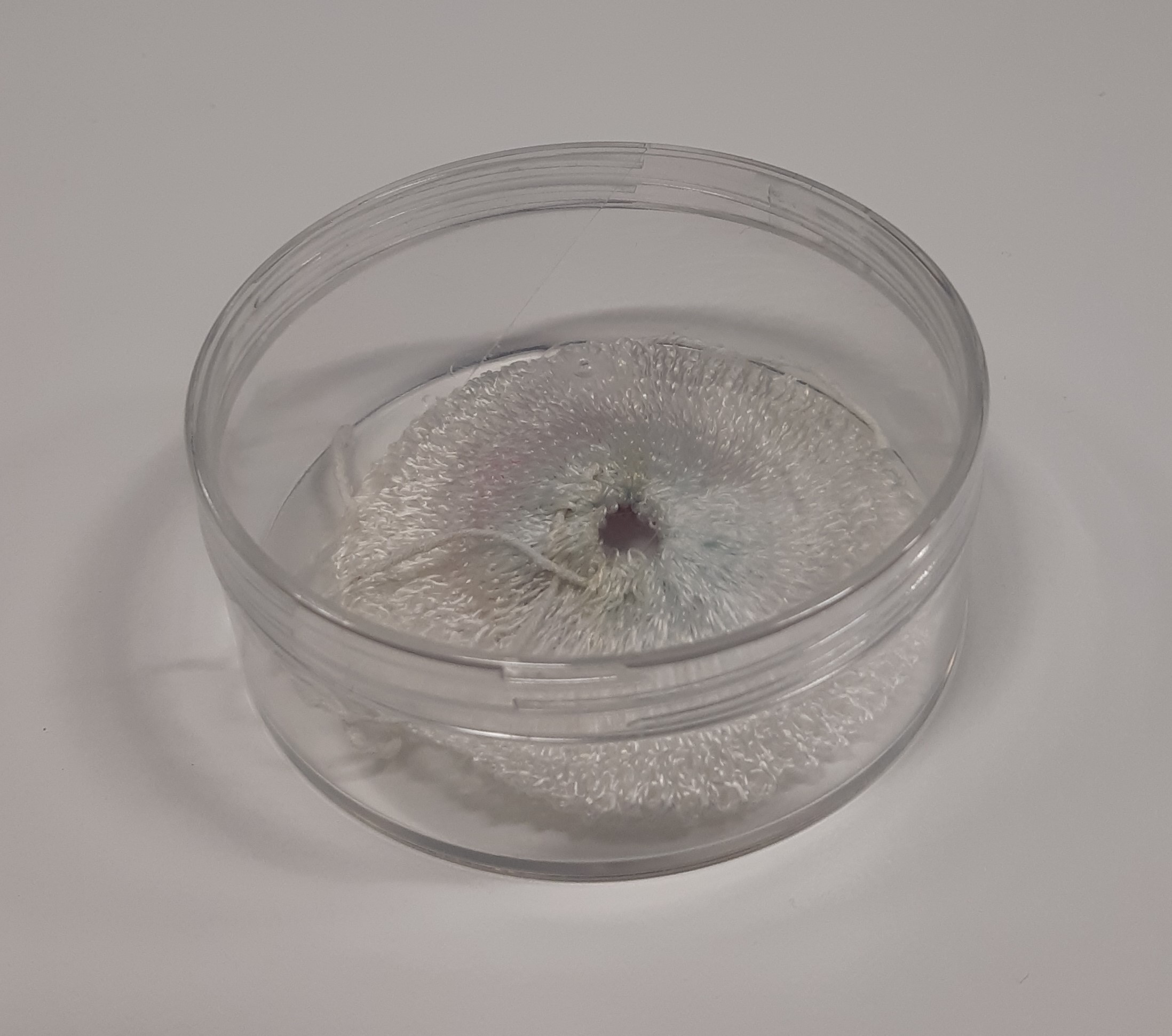
A gas mantle containing thorium-232. Activity 100 becquerels.
For more information [email protected]
Jewellery may also contain small amounts of radioactive substances. Gemstones can be irradiated with either gamma or neutron radiation to produce more beautiful and deeper colours. Neutron radiation creates radioactive substances in the stones. Gemstones are usually stored for a few months before sale, by which time most of the radioactivity has been decayed away.
According to the Radiation Act, radioactive materials may not be used in jewellery or accessories, such as watches. The import of such products is also prohibited.
For more information [email protected]
Brushes containing radioactive material have been manufactured to remove static electricity from lenses, films and records, for example. They use alpha radiation to ionise the air around an object charged with static electricity. Polonium-210 is used as the active ingredient, bound in ceramic microspheres or in metal foil at a concentration of about 20 megabecquerel. Polonium has a half-life of 138 days, which gives the brushes an effective lifetime of about one year.
The antistatic brushes do not cause any significant radiation exposure to their users, but the polonium contained in them must be considered in waste management.


Antistatic polonium brushes, which have been used for dust removal from records. Used by the film industry in the 1950s and 1960s.
For more information [email protected]
Health salts contain potassium-40 because some of the sodium in the salt has been replaced by potassium and magnesium. A small proportion of the potassium is always potassium-40. Very large quantities of potassium salts could expose the body to external radiation, but the quantities of salt in households and retail stores are so small that the external radiation they emit is indistinguishable from background radiation. The use of salt does not increase the internal radiation exposure to potassium-40 because excess potassium is not stored in the body due to the potassium balance maintained by the cells.
For more information [email protected]
Radioactive substances were added to some consumer products in the belief of imaginary health benefits, especially in the 1920s. For example, uranium, thorium and radium were used as additives in health drinks and skin care products. Sanitary pads, pillows and sanitary towels may have contained the mineral monazite with thorium. Historical objects, such as radon concentration devices or radon pads, contained a source of radium used to produce radon.
For more information [email protected]
The electrodes of TIG (tungsten inert gas arc welding) equipment may contain thorium. Torium facilitates arc ignition, stabilises the electric current and extends the life of the electrode. TIG welding electrodes can be used in concentrations of 0.4% to 4% thorium. Weld seams made with TIG welding equipment and thorium electrodes do not contain thorium. TIG welding equipment is used professionally and does not cause radiation exposure to the general public. In the workplace, exposure is low and below reference levels when the radioactivity of thorium-containing electrodes is considered in the operating procedures.
External radiation exposure from thorium-containing welding electrodes is very low, but when used carelessly, dust, in particular from electrode use, causes internal exposure through inhalation. Inhalation exposure can be reduced by good ventilation of the welding site, target air extraction, and the use of a respirator.
Good working and hygiene practices can ensure that dust or abrasive waste from handling thorium-containing electrodes is not spread, e.g. on hands, clothing or waste.
Good practices include washing hands when leaving the workplace, not eating, drinking or smoking at the workplace, and wearing appropriate protective equipment and work clothing. In addition, only tools and equipment intended for use with thorium-containing electrodes should be used.
Waste from the use of thorium electrodes is mixed waste and discarded thorium electrodes or their residues should not be recycled as metal.
Storage conditions can reduce external exposure, for example by not storing electrodes in pockets, keeping the number of electrodes stored to a minimum and storing electrodes in a dedicated place which is not used for working for long periods of time. Welding rods should also be labelled to indicate their radioactivity.
Wherever possible, the use of non-thorium-containing welding rods is recommended.
For more information [email protected]
Natural thorium, a radioactive element, has been used in the lenses of cameras and binoculars. Torium improves the optical properties of lenses. Lenses containing thorium do not cause any radiation exposure during normal use of cameras and binoculars. Private individuals can dispose individual products containing thorium lenses as mixed waste if necessary.
In the porcelain industry, uranium and thorium salts have been used to produce certain colour tones. Today, the use of these dyes has been almost completely abandoned. Uranium compounds were used as dyes and to provide a fluorescent surface in the ceramics and glass industries as early as the 1830s. Glazes could be coloured black, brown, yellow, green or red. The use of uranium was restricted in the 1940s and replaced by cheaper dyes.
Some old vases or pots may contain a significant proportion of natural uranium in the glass body or glaze, for example in yellow and green glassware. Uranium content in glassware is generally between 0.1 and 1.5 percent and most concentrations are less than 0.5 percent, although in the early 20th century it could be as high as 25 percent.
Uranium salts have also sometimes been used as colouring agents in glazed bricks, ceramic tiles and glass bricks.
From the point of view of radiation protection, uranium glass in decorative objects is of little importance, as decorative objects are hardly ever handled. The external radiation from uranium glass is generally indistinguishable from background radiation, but handling of glassware can expose the skin to very low levels of beta radiation. Since radiation exposure should be kept to a minimum, the use of glassware containing uranium is not recommended for food storage.
Uranium salts may also have been used as a colouring agent in enamelled jewellery and other enamelled objects. From a radiation protection point of view, the problem is negligible, as such antiques are also rarely handled. Jewellery dyed with uranium-containing dyes should not be used on the skin. If a private person has to dispose of uranium glassware or small enamelled objects, they are disposed of as mixed waste.


A ceramic plate made in Finland in the 1960s, the surface colour of which is obtained by using uranium-containing salt in the coating. The yellow colour of the glass vase comes from uranium. Uranium glass glows when illuminated by UV light.
For more information [email protected]
Natural radioactivity (potassium, uranium, thorium and their decay products) may be present in higher concentrations than average in products such as potassium and phosphate fertilisers and building materials. Potassium fertilisers and potassium salts emit radiation due to the presence of potassium-40, which is naturally always present as part of the potassium. Train wagons or trucks carrying potassium-containing materials are detected at border crossing radiation monitoring stations because of the large volumes of material. The use or handling of potassium fertilisers does not result in exposures above the reference level, and the use of fertilisers does not significantly increase the natural level of radioactivity in farmland.
Building materials may have been made from rocks or ashes, which have natural radionuclides from the decay series of uranium or thorium, or cesium-137 from the Chernobyl fallout in higher levels than average. Cesium-137 is present in small quantities in domestic wood and also in some imported wood. Cesium-137 can accumulate in wood ash, but the amount of cesium-137 in wood is small. Sometimes cesium-137 can be detected from large loads of timber by Customs measurements at border stations, although activity levels are low.
More information on the radioactivity of timber can be found in STUK's publication.
Radioactivity of timber in Finland: the environmental radiation monitoring action programme (In Finnish, Julkari.fi)
The Radiation Act requires the radioactivity of construction products to be determined if they contain certain stone materials, ash or mineral-based raw material.
Refractory materials, such as firebricks, may also contain natural radioactive substances originating from the minerals used in their manufacture.
Further information: Radioactive substances in construction products and ash (stuk.fi)
For more infromation [email protected] (Natural Radiation Regulation)
Scrap yards and dismantling yards have radiation detection equipment, as radiation sources can sometimes accidentally end up among scrap metal. Elevated dose rates due to caesium-137 can also sometimes be detected in materials contaminated by the Chernobyl fallout, e.g. in old roof tiles.
In principle, caesium-137 from the Chernobyl fallout can be present in small quantities in, for example, goods and furniture from the fallout area. However, the radioactivity in these items is generally low, at background levels.
For more information [email protected]
Radiating items or materials that may have natural radioactive substances accumulated, from the decay series of uranium or thorium can also be found from scrap metal. These can also be detected, for example at scrap yards, using radiation gates or radiation meters, if the radiation from the materials can be distinguished from the background radiation. For example, in groundwater treatment, ore enrichment or the pigment industry, precipitates containing natural radioactive substances may accumulate in parts of the process equipment.
For more information [email protected] (Natural Radiation Regulation)
Amateur geologists or rock collectors may have rock or ore samples with uranium or thorium minerals in their collections. It is always advisable to mark well any rocks that are emitting radiation and to take care that the material that crumbles from them does not spread unintentionally and thus cause radiation exposure.
For the purposes of the Nuclear Energy Act, uranium ore is defined as mineral with an average uranium content of more than 1 kg per tonne (1000 mg/kg of uranium), while thorium ore is defined as mineral with an average thorium content of higher than 30 kg per tonne, except for monazite, or higher than 100 kilograms per metric tonne for monazite. Where concentrations in ore exceed these limits, authorisations and notifications are governed by the Nuclear Energy Act. For example, the import of a uranium ore sample must be notified to the Radiation and Nuclear Safety Authority. The export of ore containing more than 1 kg of uranium requires a licence.
If the uranium and thorium content of the minerals is below the limits of the Nuclear Energy Act, the Radiation Act applies. The Radiation Act specifies that, for example, private collections of rocks or minerals are not subject to the obligation to report or notify natural radiation exposure. For commercial activities, the reporting obligation under the Radiation Act applies to activities that involve handling of materials where the activity concentration of uranium-238 or thorium-232 or their decay products exceeds 1 Bq/g. This activity level corresponds to a concentration of about 80 mg/kg for uranium and about 240 mg/kg for thorium. In such cases, the operator must inform the Radiation and Nuclear Safety Authority of their activities. This may apply, for example, to public rock or mineral collections.
For more information [email protected] (Natural Radiation Regulation)
References and additional information
- IAEA Safety Standards, Radiation Safety for Consumer Products (2016) (pdf)
- Systematic radiological assessment of exemptions for source and byproduct materials (2001) Nureg-1717 U.S. Nuclear regulatory commission (pdf)
- Radiological implications of the use of uranium in vaseline glass, Watson SJ ja Hughes JS (2010) Journal of Radiological protection, 30, 535–544 (pdf)